Chlorine Disinfection
by Matt Curtis & Erik Johnston 
Table of Contents
 History History
 Theory Theory
 Implementation Implementation
 References References
History
Throughout the history of water treatment, chlorination has been synonymous
with disinfection. Chlorination was the first method used to disinfect water in
approximately 1825 and remained the only widely used method for over 50 years.
The following timeline shows a brief history of chlorination in water and
wastewater treatment over the past 100 years. The graph to the right of the
timeline shows the effect of filtration and then chlorine disinfection on the
number of typhoid cases. Filtration dramatically reduced the number of cases of
typhoid, and chlorine then also further reduced the number of cases (Data from
Davis, 1991.).
TIMELINE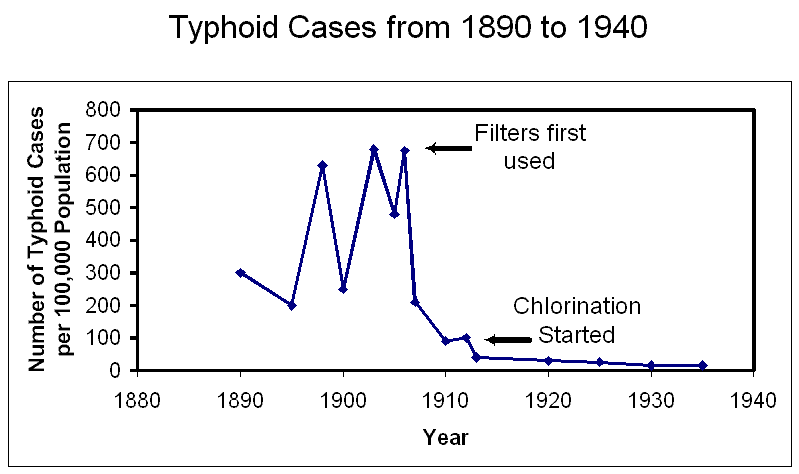 
 1879 1879
 1893 1893
 1903 1903
 1908 1908
 1910 1910
 1912 1912
 1913 1913
 1914 1914
 1919 1919
 1925 1925
 1939 1939
 1960 1960
 1972 1972
1879- This marked the first time that chlorine was applied as a
disinfectant. William Soper of England treated the feces of typhoid patients
before disposal into the sewer. He used chlorinated lime, which was a common
form of chlorine used initially. (White, 1972) Return
to timeline.
1893- This date was the first time that chlorine was applied as a
disinfectant on a plant scale basis. This application was made at Hamburg,
Germany. (White, 1972) Return
to timeline.
1903- This marked the first time chlorine gas was used as a
disinfectant in drinking water. This took place in Middlekerke, Belgium. Prior
to this date, chlorine was applied through the use hydrated lime, chloride of
lime, or bleaching powder. The use of chlorine gas was designed by Maurice Duyk,
a chemist for the Belgian Ministry of Public Works. (Pontius, 1990) Return
to timeline.
1908- The first full scale chlorine installation at a drinking water
plant in the United States was initiated in this year. This installation took
place at the Bubbly Creek Filter Plant in Chicago. This plant served the Chicago
Stockyards and was designed by George A. Johnson. The raw water contained a
large amount of sewage which was causing sicknesses in the livestock. Johnson
implemented chlorine through chloride of lime, and the bacterial content of the
water dropped drastically. (Pontius, 1990)Return
to timeline. 
1910- C. R. Darnall became the first to use compressed chlorine gas
from steel cylinders which is an approach still commonly used today. His
installation was in Youngstown, Ohio. His implementation used a
pressure-reducing mechanism, a metering device, and an absorption chamber. It
was moderately successful, but his setup was only used once. The photo to the
right is typical of modern design. (White, 1972) (Photo by Matt Curtis)Return
to timeline.
1912- John Kienle, chief engineer of the Wilmington, Delaware water
department, invented another way to apply chlorine to drinking water. He
developed a way to push compressed chlorine from cylinders into an absorption
tower in which water was flowing opposite the flow of the chlorine. Because the
gas flow was opposite the water flow, the chlorine was able to disinfect the
water. (Pontius, 1990)Return
to timeline.
1913- An Ornstein chlorinator was installed at Kienle's Wilmington,
Delaware water treatment plant. This marked the first time a commercial
chlorination system was installed at a municipal water treatment plant. The
chlorinator used the same basic premise that Kienle's previous installation did,
but the Ornstein chlorinator used both a high and low pressure gauge to more
accurately control the amount of chlorine added to the system. (Pontius, 1990)
Return
to timeline. 
1914- On October 14, 1914, the Department of the Treasury enacted the
first set of standards that required the use of disinfection for drinking water.
These standards called for a maximum level of bacterial concentration of 2
coliforms per 100 millilters. Because chlorination was the main disinfectant at
the time, these standards dramatically increased the number of treatment plants
using chlorine. (White, 1972) Return
to timeline.
1919- Two important discoveries were made during this year. Wolman and
Enslow discovered the concept of chlorine demand which states that the amount of
chlorine needed to disinfect the water is related to the concentration of the
waste and the amount of time the chlorine has to contact the water. The other
important discovery of 1919 was by Alexander Houston. He discovered that
chlorine can also eliminate taste and odor problems in water. (Pontius, 1990) Return
to timeline.
1925- New drinking water standards were enacted that reduced the
maximum permissible limit of coliforms from 2 to 1 coliform per 100 millilters.
This increased the amount and frequency of chlorination again. (White, 1972) Return
to timeline.
1939- The theory of the chlorine breakpoint was discovered in this
year. Chlorine breakpoint theory is discussed in the following section. (White,
1972) Return
to timeline.
1960- A new implementation practice was discovered in this year. The
compound loop principle of chlorinator control was implemented, which is the
most recent major discovery in chlorine application. (White, 1972) Return
to timeline.
1972- A report entitled "Industrial Pollution of the Lower Mississippi
River in Louisiana" was published containing the first evidence of disinfection
byproducts in drinking water resulting from organic pollution in source water.
(Pontius, 1990) Return
to timeline.

As is evident by the dates in the timeline, most of the innovation in
chlorination occurred over 70 years ago. Very few innovations or discoveries
have been made recently. Most of the current research is being performed in
other areas of disinfection. These areas include ozone, chlorine dioxide, and UV
radiation. Chlorine is still the most widely used disinfectant in the United
States, but other areas of the world are beginning to use other methods of
disinfection with increasing frequency. Since chlorine is still widely used, a
thorough understanding of how it disinfects and is implemented is important to
those interested in water treatment. Return
to Table of Contents.
Theory
Disinfection with chlorine is very popular in water and wastewater treatment
because of its low cost, ability to form a residual, and its effectivness at low
concentrations. Although it is used as a disinfectant, it is a dangerous and
potentially fatal chemical if used improperly.
Despite the fact the disinfection process may seem simple, it is actually a
quite complicated process. Chlorination in wastewater treatment systems is a
fairly complex science which requires knowledge of the plant's effluent
characteristics. When free chlorine is added to the wastewater, it takes on
various forms depending on the pH of the wastewater. It is important to
understand the forms of chlorine which are present because each has a different
disinfecting capability. The acid form, HOCL, is a much stronger disinfectant
than the hypochlorite ion, OCL-. The graph below depicts the chlorine fractions
at different pH values (Drawing by Erik Johnston). 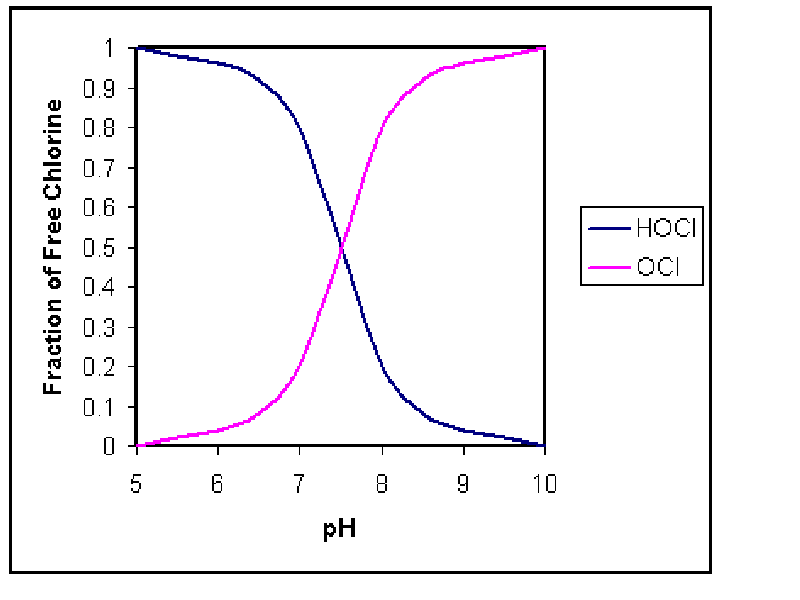
Ammonia present in the effluent can also cause problems as chloramines are
formed, which have very little disinfecting power. Some methods to overcome the
types of chlorine formed are to adjust the pH of the wastewater prior to
chlorination or to simply add a larger amount of chlorine. An adjustment in the
pH would allow the operators to form the most desired form of chlorine,
hypochlorus acid, which has the greatest disinfecting power. Adding larger
amounts of chlorine would be an excellent method to combat the chloramines
because the ammonia present would bond to the chlorine but further addition of
chlorine would stay in the hypochlorus acid or hypochlorite ion state.
a) Chlorine gas, when exposed to water reacts readily to form hypochlorus
acid, HOCl, and hydrochloric acid. Cl2 + H2O -> HOCl +
HCl
b) If the pH of the wastewater is greater than 8, the hypochlorus acid will
dissociate to yield hypochlorite ion. HOCl <-> H+ +
OCl-- If however, the pH is much less than 7, then HOCl will not
dissociate.
c) If ammonia is present in the wastewater effulent, then the hypochlorus
acid will react to form one three types of chloramines depending on the pH,
temperature, and reaction time.
Monochloramine and dichloramine are formed in the pH range of 4.5 to 8.5,
however, monochloramine is most common when the pH is above 8. When the pH of
the wastewater is below 4.5, the most common form of chloramine is trichloramine
which produces a very foul odor. The equations for the formation of the
different chloramines are as follows: (Reynolds & Richards, 1996)
Monochloramine: NH3 + HOCl -> NH2Cl +
H2O
Dichloramine: NH2Cl + 2HOCl -> NHCl2 +
2H2O
Trichloramine: NHCl2 + 3HOCl -> NHCl3 +
3H2O
Chloramines are an effective disinfectant against bacteria but not against
viruses. As a result, it is necessary to add more chlorine to the wastewater to
prevent the formation of chloramines and form other stronger forms of
disinfectants.
d) The final step is that additional free chlorine reacts with the chloramine
to produce hydrogen ion, water , and nitrogen gas which will come out of
solution. In the case of the monochloramine, the following reaction occurs:
2NH2Cl + HOCl -> N2 + 6HCl +
H2O
Thus, added free chlorine reduces the concentration of chloramines in the
disinfection process. Instead the chlorine that is added is allowed to form the
stronger disinfectant, hypochlorus acid.
Perhaps the most important stage of the wastewater treatment process is the
disinfection stage. This stage is most critical because it has the greatest
effect on public health as well as the health of the world's aquatic systems. It
is important to realize that wastewater treatment is not a cut and dry process
but requires in depth knowledge about the type of wastewater being treated and
its characteristics to obtain optimum results. (White, 1972) 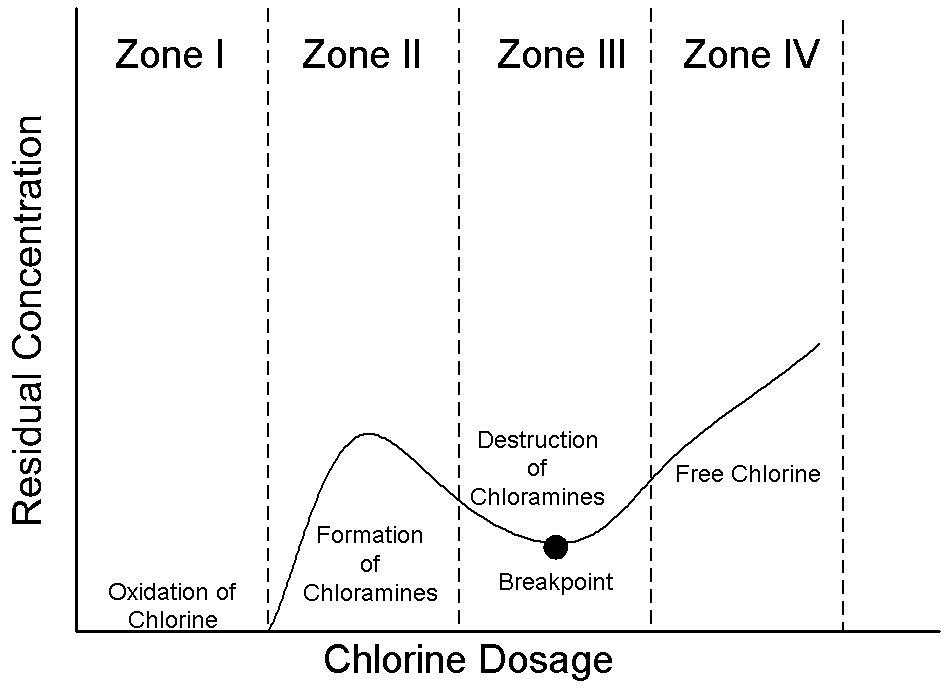
The graph shown above depicts the chlorine residual as a function of
increasing chlorine dosage with descriptions of each zone given below (Drawing
by Erik Johnston, adapted from Reynolds and Richards, 1996).
· Zone I: Chlorine is reduced to chlorides.
· Zone II: Chloramines are formed.
· Zone III: Chloramines are broken down and converted to nitrogen gas which
leaves the system (Breakpoint).
· Zone IV: Free residual.
Therefore, it is very important to understand the amount and type of chlorine
that must be added to overcome the difficulties in the strength of the
disinfectant which results from the wastewater's characteristics. Return
to Table of Contents.
Implementation
Water Treatment
The following is a schematic of a water treatment plant (Drawing by Matt
Curtis). 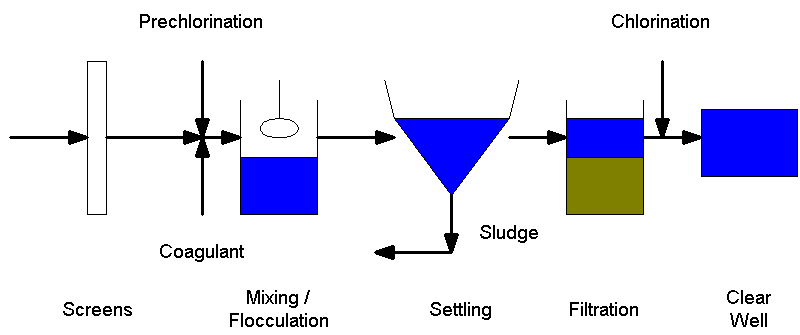
In water treatment, pre-chlorination is utilized mainly in situations where
the inflow is taken from a surface water source such as a river, lake, or
reservoir. Chlorine is usually added in the rapid mixing chamber and effectively
prevents the majority of algal growth. Algae is a problem in water treatment
plants because it builds up on the filter media and increases the head which
means that the filters need to be backwashed more frequently. In addition, the
algal growth on the filter media causes taste and odor problems in the treated
water. (Reynolds & Richards, 1996) 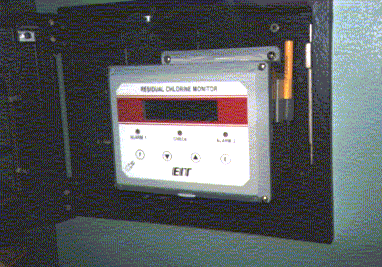
In the picture to the left, a residual monitor checks the chlorine level in
the water leaving the treatment plant. A minimum value is required to prevent
regrowth of bacteria throughout the distribution system, and a maximum value is
established to prevent taste, odor, and health problems (Photo by Matt Curtis).

Post chlorination is almost always done in water treatment, but can be
replaced with chlorine dioxide or chloramines. In this stage chlorine is fed to
the drinking water stream which is then sent to the chlorine contact basin to
allow the chlorine a long enough detention time to kill all viruses, bacteria,
and protozoa that were not removed and rendered inactive in the prior stages of
treatment (Photo by Matt Curtis).
Drinking water requires a large addition of chlorine because there must be a
residual amount of chlorine in the water that will carry through the system
until it reaches the tap of the user. After post chlorination, the water is
retained in a clear well prior to distribution. In the picture to the right, the
clear pipe with the floater designates the height of the water within the clear
well. (Reynolds & Richards, 1996) Return
to Table of Contents.
Wastewater Treatment
The following is a schematic of a wastewater treatment plant (Drawing by Erik
Johnston). 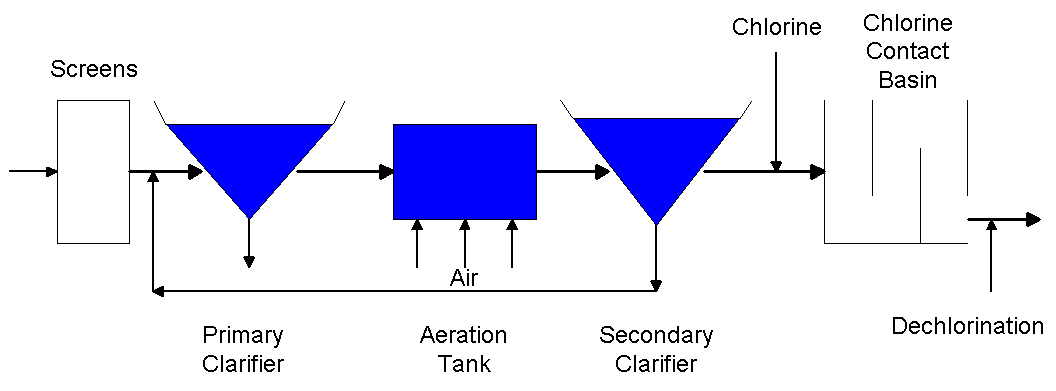
The final stage of the wastewater treatment process is chlorination, which is
used to disinfect the wastewater before it is returned to a river or body of
water. Disinfection is necessary to kill any pathogens that may have survived
the treatment process and could cause harm to humans or aquatic wildlife. In
this stage of the treatment process, chlorine is typically fed into the
wastewater stream as it flows to the chlorine contact basin. The chlorine
contact basin is a baffled basin which allows the chlorine additional time to
react with the wastewater to kill the pathogens.
The following is an animation of a drop of water as it passes through a
chlorine contact basin. 
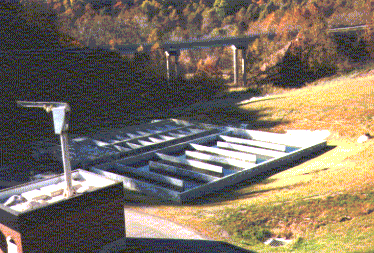
The photograph to the left is of the chlorine contact basin at the Blacksburg
Wastewater Treatment Plant. (Photo by Matt Curtis)
Following the chlorine contact basin, the wastewater stream is fed a
chemical, typically sulfur dioxide, to dechlorinate the water. Dechlorination is
essential because chlorine is toxic to aquatic life. The final stage after
dechlorination is to again oxygenate the water before it is emitted to the
stream so it will contain enough dissolved oxygen to support the aquatic life.
This stage of the process is typically done by allowing the wastewater stream to
flow over a series of weirs before it is emitted to the water body. (Reynolds
& Richards, 1996)
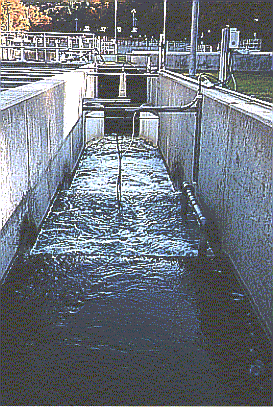
The photograph to the right is of the discharge from the chlorine contact
basin. The inlets for the sulfur dioxide addition can be seen as pipes hanging
into the water. The turbulent flow of the water is caused by the submerged weirs
which oxygenate the water. Also shown are wires for equipment that will monitor
the properties of the water as it leaves the chlorine contact basin. (Photo by
Matt Curtis) Return
to Table of Contents.
References
1. Davis, Mackenzie, L. Introduction to Environmental Engineering
McGraw-Hill, Inc. NY, NY. 1991.
2. Pontius, Frederick, Ed. Water Quality and Treatment, 4th Ed.
American Water Works Association. McGraw-Hill, Inc. NY, NY. 1990.
3. Reynolds, Tom, Paul Richards. Unit Operations and Processes in
Environmental Engineering, 2nd Ed. PWS Publishing Company. Boston. 1996.
4. White, Clifford. Handbook of Chlorination. Van Nostrand Reinhold
Company. NY, NY. 1972.
Enlaces a otras páginas relacionadas Return
to Table of Contents.
Send comments or suggestions to:
Student Authors: Matt Curtis & Erik
Johnston
Faculty Advisor: Daniel Gallagher, dang@vt.edu
Copyright © 1997 Daniel
Gallagher
Last Modified: 2-14-1998 |